Transport of molecular motors into cilia
Molecular motors produce the force that powers the beat of sperm cell tails to generate movement toward the egg cell for fertilization. New research now shows how the molecular motors that power the movement of sperm cells are recognized and specifically transported into the tail region of the cell. This knowledge can pave the way for a better understanding of diseases causing mutations that cause sterility.
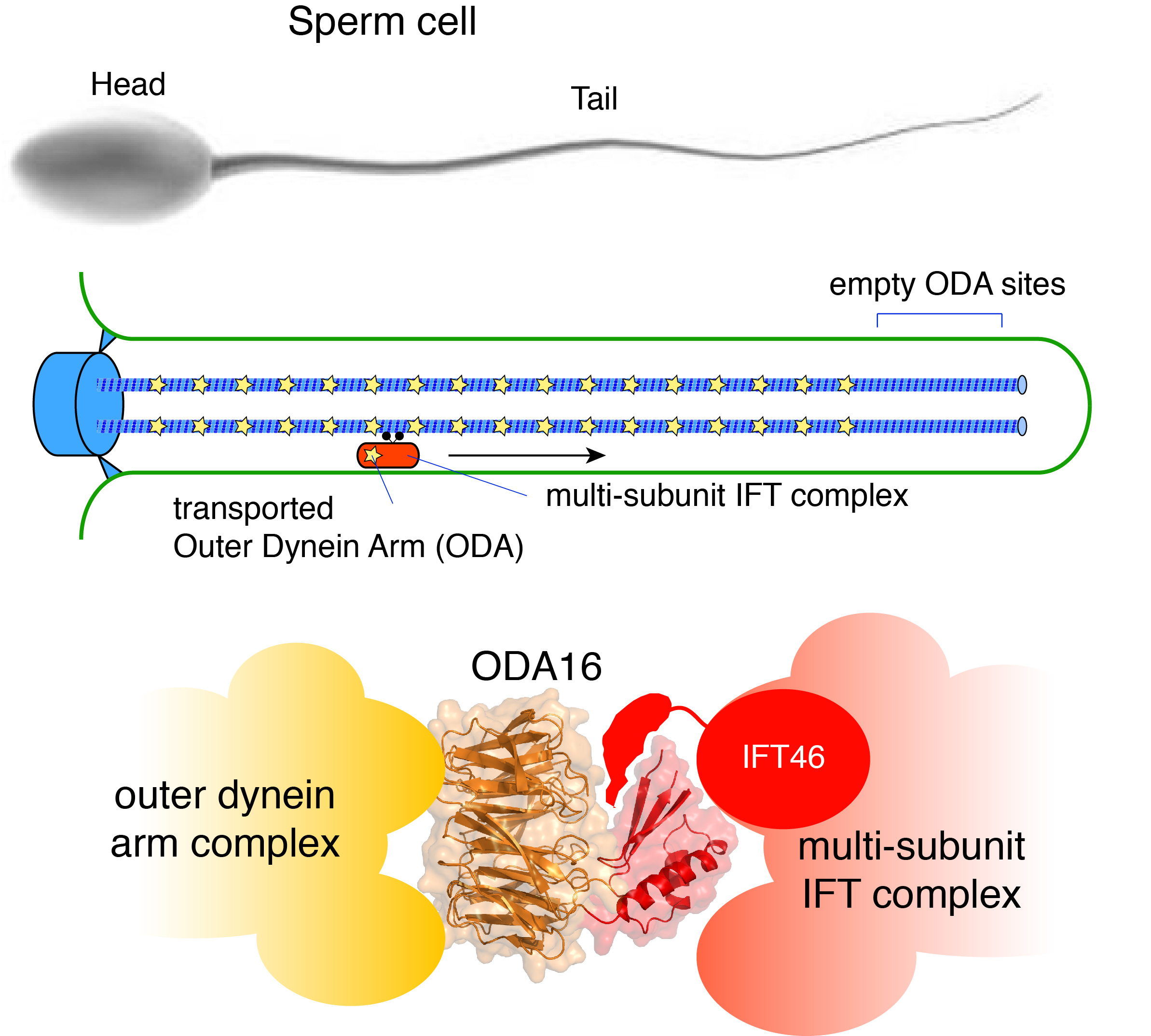
Molecular motors use the molecule ATP as energy source to organize the inner life of cells. Dyneins are the largest and most complex molecular motors and are responsible for intracellular transport and for generation of the force required for motility of cilium organelles. Cilia are thin structures found on the surface of our cells where they function as sensors receiving signals from the environment and as motors causing the cell or the environment to move.
Motile cilia are found as a single copy on sperm cells and in multiple copies on cells in our lungs where they generate a fluid flow necessary for the removal of dust particles and pathogens from the airways. The large dynein motors (known as ‘outer dynein arms´, ODA) - that are necessary for the motility of cilia - are actively transported into cilia via the intraflagellar transport (IFT) system and the transport adaptor ODA16. Mutations in dynein motors or IFT factors can result in infertility and respiratory deficiency.
An international research team now mapped how dynein motors are recognized by the adaptor protein ODA16 and imported into cilia via the IFT system. The crystal structure of ODA16 shows how the largest barrel-like domain recognizes dynein motors and simultaneously binds the IFT complex via a cleft generated by the barrel domain and a smaller domain located on top of the barrel. ODA16 thus functions as a true adaptor between the large dynein and IFT complexes (see figure).
This new knowledge can pave the way for structure determination of IFT complexes associated with dynein motors via ODA16, which will lead to a deeper understanding of ciliary mechanisms and disease causing mutations in genes encoding dynein and IFT proteins.
The research team consists of Michael Taschner and Esben Lorentzen from the Department of Molecular Biology and Genetics, Aarhus University, Jérôme Basquin from the Max Planck Institute, Andre? Moura?o from the Helmholz Center (both in Munich, Germany) and Mayanka Awashti from Maryland University, USA.
The results are published in the scientific Journal of Biological Chemistry.
For further information, please contact
Associate Professor Esben Lorentzen
Department of Molecular Biology and Genetics
Aarhus University, Denmark
el@mbg.au.dk - +45 8715 5478
